What is HS?
Heparan sulfate (HS) are highly sulfated polysaccharides that reside on the cell surface and extracellular matrix of all mammalian cell types. HS is assembled in the Golgi apparatus by initially forming a linear polymer composed of alternating D-glucuronic acid (GlcA) and N-acetyl-D-glucosamine (GlcNAc) moieties (Fig. 1), that are modified by a series of enzymatic reactions involving N-deacetylation/N-sulfation, epimerization of GlcA to L-iduronic acid (IdoA), and O-sulfation of the C-2 hydroxyl of IdoA and the C-6 and C-3 hydroxyl of glucosamine moieties. Often the modifications proceed partially resulting in as many as twenty different disaccharide moieties. These disaccharides can be arranged in different sequences creating specific epitopes that can recruit a multitude of regulatory proteins. The interaction between HS and these proteins is critical for many biological processes, and for example it coordinates complex signaling pathways controlling stem cell self-renewal, as well as cell fate, proliferation or differentiation to specific lineages, such as midbrain dopaminergic neurons (mDA neurons).
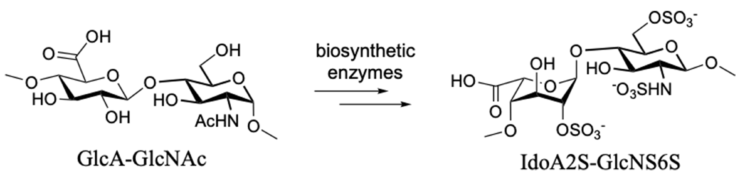
Fig. 1. Biosynthesis of HS. Initially, a polymer is formed composed of GlcA-GlcNAc repeating units, which is extensively modified by epimerization and sulfation. These modifcations are incomplete generating considerable molecular complexity.
HS in Parkinson’s disease
Alterations in HS expression are associated with many diseases, such as cancer. In addition, HS is critical for stem cell regulation. The degeneration of mDA neurons is one of the major pathogenic mechanisms in Parkinson’s disease (PD). Alterations in the levels or function of HS-biosynthetic enzymes, such as sulfatases, lead to changes in HS structures, which in turn will influence the recruitment of morphogens that control Wnt-β-catenin and Wnt-planar cell polarity signalling, which in turn control the generation of mDA neurons. While it is known that the balance between these two pathways is essential for correct development of mDA neurons, activation of Wnt-PCP or their modulation with HS remains to be implemented in hPSC protocols for PD cell replacement therapy.
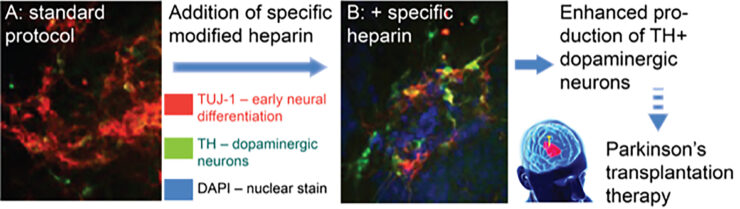
Fig. 2. Specific heparins enhance dopaminergic neuron (DA) generation. Mouse embryonic stem cells treated with a specific modified heparin demonstrate a marked increase in proportion of DA neurons (tyrosine hydroxylase, TH+), compared to tubulin (TUJI-1, an early neural differentiation marker).
Sequencing issues
Even though it is clear that HS play key roles in health and disease, we know little about how they actually perform their functions. Heparin/HS possess unparalleled levels of structural complexity compared to other classes of biomolecules, such as proteins or DNA, and often occur in many isomeric forms that cannot be resolved by current analytical methods. A commonly applied analytical approach is based on hydrolysis using lysates followed by identification of the resulting disaccharides by HPLC. While this method provides compositional information, it does not reveal how disaccharide motifs are organised into functional HS epitopes. Another method is mass spectromy (MS) of HS saccharides. This method can provide compositional information, such as chain length, degree of sulfation and number of acetyl groups, however it does not provide sequence information, and in particular cannot reveal iduronic acid epimerization or the modification of sulfate and acetyl moieties. Although some advances have been made, numerous difficulties exist for MS/MS-based sequencing of HS saccharides, among which sulfate loss during fragmentation is a major obstacle. As a result, less then 20 HS sulfation sequences have been fully defined to date.